Authors
- Ferrari, Virginia (1); ibáñez, Facundo (1, 2); Cabrera, Danilo (2); Pintado, Betiana (3).
- iNSTiTUTO NACiONAL DE iNVESTiGACiÓN AGROPECUARiA, iNiA.PLATAFORMA DE AGROALiMENTOS. MONTEViDEO, URUGUAY.
- iNSTiTUTO NACiONAL DE iNVESTiGACiÓN AGROPECUARiA, iNiA.PROGRAMA DE iNVESTiGACiÓN EN PRODUCCiÓN FRUTÍCOLA. MONTEViDEO, URUGUAY.
- CONSEjO DE EDUCACiÓN TÉCNiCO PROFESiONAL, UTU. MONTEViDEO, URUGUAY.
En este trabajo se evaluaron accesiones de frutos nativos de ‘Arazá’ (Psidium cattleianum), ‘Guaviyú’ (Myrcianthes pungens) y Guayabo del país’ (Acca sellowiana) como parte de un programa de selección de variedades para obtener cultivares comerciales. El objetivo es ofrecer a los productores nuevas posibilidades de cultivos frutícolas locales y brindar a los consumidores alternativas alimenticias con reconocidas propiedades nutricionales y nutracéuticas. En diferentes genotipos de estos frutos se evaluó la calidad fisicoquímica, el contenido de compuestos fenólicos, vitamina C y antocianinas, y la relación con la actividad antioxidante total por DPPH y ORAC. Las metodologías para determinar compuestos bioactivos y capacidad antioxidante fueron validadas a escala de microvolúmenes, siguiendo principios de “química verde”. El contenido de compuestos bioactivos difiere significativamente dependiendo de la especie y accesión (p ≤ 0,05). Los resultados encontrados confirman las propiedades nutricionales y capacidad antioxidante potencial de estos frutos. El efecto de variables agronómicas y ambientales en genotipos seleccionados debe investigarse para ajustar la tecnología de producción comercial.
Palabras clave: frutos nativos, vitamina C, compuestos fenólicos, DPPH, ORAC.
Diferentes acessos de frutos nativos de ‘Araçá’ (Psidium cattleianum), ‘Guabijú’ (Myrcianthes pungens) e ‘Goiaba Serrana’ (Acca sellowiana) foram avaliados como parte de um programa de seleção para obtenção de cultivares comerciais. O objetivo foi oferecer aos produtores a opção de cultivo de novas frutíferas locais e proporcionar aos consumidores alternativas alimentares com propriedades nutricionais e nutracêuticas reconhecidas. A qualidade físico-química, conteúdo de compostos fenólicos, vitamina C e antocianinas, e a relação com a atividade antioxidante total por meio de DPPH e ORAC foram avaliadas nos diferentes genótipos. As metodologias para definição dos compostos bioativos e capacidade antioxidante foram validadas a escala micro volumétricas, seguindo os princípios da “química verde”. O conteúdo de compostos bioativos diferiu significativamente dependendo da espécie e do acesso (p ≤ 0,05). Os resultados encontrados confirmam as propriedades nutricionais e a potencial capacidade antioxidante destes frutos. Os efeitos das variáveis agronômicas e ambientais nos genótipos selecionados devem ser investigados para ajustar as tecnologias de produção comercial e explorar as características nutracêuticas encontradas.
Palavras-chave: frutos nativos, vitamina C, compostos fenólicos, DPPH, ORAC.
Introduction
Some fruit chemical compounds such as vitamins, flavonoids, anthocyanins and other phenolic compounds can act reducing the harmful effects of oxidative stress and preventing the deterioration of physiological functions and hence the occurrence of some types of cancer, neurodegenerative and cardiovascular diseases (Chang, et al., 2016).
Regarding these compounds, several methods are used to determine their concentrations. Most of them include extraction and spectrophotometric measurements steps requiring a large amount of solvent (Harborne, 1998). Even the scientific community searches for new green and efficient techniques in order to improve the extraction process reducing the use of hazardous and/or contaminant chemicals (Bijttebier, et al., 2016). Ultrasound-assisted extraction (UAE) and microplate spectrophotometric readers are common examples of volume microscale working in these assays. Furthermore different methodologies have been employed to evaluate the in vitro antioxidant capacity that those compounds confer to the fruits, of which DPPH, ABTS, FRAP, ORAC and TEAC are the most widely used (Parsons, 2017). At least two of these assays must be combined to provide a reliable representation of the total antioxidant capacity of a foodstuff because the methods might be influenced by several factors, mainly physicochemicals, as free radicals generation (Apak, et al., 2013). The DPPH (2,2-diphenyl-1-picrylhydrazil) method involves this radical chromogen compound, which in presence of antioxidants loses its colored signal. In the sample solution, antioxidants lighten the color of DPPH by donating an electron/hydrogen atom to the unpaired electron of DPPH that turns the purple color of DPPH colorless. The reaction is monitored using a simple UV/Vis spectrophotometer. The ORAC (Oxygen Radical Absorbance Capacity) assay measure scavenging capacity of antioxidant in the sample against the peroxyl radical formed from AAPH reactive (2,2’-azobis (2-amidinopropane) dihydrochloride) and a fluorescent probe (usually fluorescein) in a competitive reaction mechanism. The antioxidant elimination of peroxyl radical by irreversible reaction protects the fluorescein from degradation. The decay in fluorescence due to the attack of radicals and protection by antioxidant results in a curve. Calculating the area under the fluorescence decay curve (AUC) according to different concentrations of a standard compound (usually Trolox; (±)-6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid, a water-soluble analogue of vitamin E), a calibration equation is achieved and can be used to evaluate the radical cleansing activity of antioxidants compounds in the sample. ORAC is the standardized and actually adopted method to determine antioxidant capacity in food (Wu, et al., 2004; Prior, et al., 2005; Prior, 2015).
In the native flora of Uruguay there are more than 20 species that produce edible fruits. The predominant species include those belonging to the family Myrtaceae: ‘Guayabo del país’ (Acca sellowiana (Berg.) Burret); ‘Arazá’ (Psidium cattleianum Sab.) and ‘Guaviyú’ (Myrcianthes pungens (Berg.) Legr.). Multidisciplinary investigations have been developed for years to prospect, characterize and select promising genetic materials (Cabrera, et al., 2008; Feippe, et al., 2011). Although these fruits are not common in the retail market, the interest in planting, commercialize and consume them has increased recently, not only because their unique flavor and aroma but also for their outstanding nutraceutical properties given by the high content of antioxidant compounds (Pasquariello, et al., 2015; Seraglio, et al., 2018).
The aim of this study was to determine the bioactive compounds concentration and antioxidant activities of three native fruits species using the DPPH and ORAC methods and to supply more information on the genetic diversity of the evaluated selections.
Materials and methods
Samples
The fruits were harvested from an evaluation orchard at INIA “W. Ferreira Aldunate” Experimental Station. Fruits from seven selections of ‘Arazá’ (AA - P. cattleyanum f. lucidum), three of ‘Guaviyú’ and two of ‘Guayabo del país’ were randomly hand-harvested at the commercial maturity stage. Samples were 1 kg (Arazá and Guaviyú) and 2 kg (Guayabo del país). In the laboratory the fruits were selected based on the uniformity, appearance and the absence of physical damage, and weight and moisture were determined. Three samples of 20 fruits for each accession were selected. ‘Guayabo del país’ fruits were peeled and ‘Guaviyú’ seeds were removed. The edible portions of the fruits were homogenized using a blender during 1 min. After centrifugation at 12500 rpm during 10 min, a small amount of homogenate was used for physicochemical analyses and the remainder immediately frozen at -80 ℃ until the analysis of total phenolics, vitamin C, anthocyanins and antioxidant activities.
Physicochemical parameters
The pH was determined using a digital pHmeter (MM374, Hach). Titratable acidity (TA) was determined by titration with NaOH 0,1 M and expressed as grams of citric acid in 100 mL of fruit sample. The total soluble solids (SS) were determined using a manual refractometer (RHB-90, ATC) and expressed as ºBrix.
Bioactive compounds content
Bioactive compounds were extracted from 0,2 g of fresh fruit homogenates using 1 mL of methanol/water (80:20, v/v). After homogenization, the samples were placed in an ultrasonic bath during 10 min at 20 ℃ and stored for 4 h at 20 ℃ avoiding light exposure. Then, they were centrifuged at 12500 rpm during 5 min. The supernatant was diluted for subsequent composition and antioxidant analyses. All spectrophotometrical data were obtained using a 96 well plate multi-mode microplate reader with two independent dispensers (Synergy HT1, BioTek Instruments Inc.).
Anthocyanins content was measured using a pH-differential protocol (Giusti and Wrolstad, 2001) with some modifications. An aliquot of 40 mL of methanolic extract was placed in the microplate and diluted with 200 mL of potassium chloride (0,025 M; pH 1,0) or sodium acetate (0,4 M; pH 4,5) buffers. Absorbance was measured at 520 and 700 nm. The absorption (A) of the anthocyanins in the diluted samples was calculated from the Equation 1 and anthocyanins concentration was calculated using the cyanidin 3-glucoside extinction coefficient (26.900 M-1cm-1).
$$A = (A_{520}\text{-}A_{700})\thinspace _{pH\thinspace 1.0}\thinspace \text{-}\thinspace (A_{520}\text{-}A_{700})\thinspace _{pH\thinspace 4.5}$$ (Eq. 1)
The results were expressed as mg cyanidin 3-glucoside (mg c3g) per 100 g of fresh fruit weight (FW).
Ascorbic acid (vitamin C) and total phenolic contents were estimated following the method of Sánchez-Rangel et al. (2013). Briefly, 15 mL of methanolic extract was diluted with distilled water in a microplate well and the Folin-Ciocalteu reagent was added. The mixture was agitated, incubated for 3 min and the absorbance at 765 nm determined. The vitamin C concentration was estimated against ascorbic acid standard curve and expressed as milligrams of ascorbic acid (aa) per 100 g of fresh fruit weight (FW). To obtain the total phenolic content in the sample, the assay was continued by adding Na2CO3 to the mixture present in the microplate wells and incubated for 2 h at room temperature in the dark. Then the absorbance at 765 nm was measured and values compared against gallic acid standard calibration curve. The results were expressed as milligram of gallic acid equivalents (GAE) per 100 g of fresh fruit weight (FW).
Antioxidant activity
The Brand-Williams et al. (1995) assay slightly modified was used to evaluate the antioxidant capacity of extract based on the quantification of free radical-scavenging of DPPH. Briefly, 15 mL of appropriate diluted methanolic extract was mixed with 250 mL DPPH solution (125 µM) in methanol/water (80:20, v/v). The mixture was shaken and incubated for 24 h at 20 ℃ in the dark. The reduction of absorption was measured at 517 nm and quantified using a Trolox standard curve. The results were expressed as mmol Trolox equivalents (TE) per 100 g of fresh fruit weight (FW).
For ORAC, the procedure followed the methods of Ou et al. (2002) and Prior et al. (2005) with some modifications. Diluted fruit methanolic extracts or control (gallic acid) or standards solutions of Trolox (25 mL) were transferred to microplates. The plates were placed in the microplate reader above described and 150 mL of fluorescein solution in 75 mM potassium phosphate buffer (0,008 µM) were added using the automatic dispenser. After shake and incubation at 37 ℃ for 30 min, 25 mL AAPH (153 mM) prepared freshly with 75 mM phosphate buffer was added to each well. The plate was agitated and fluorescence was measured every minute for 60 min. The filters were set at 485 nm and 528 nm for excitation and emission, respectively. The results of each sample were estimated based on standard curve of Trolox concentration and net area in the fluorescence decay curve. The ORAC activity was expressed as mmol Trolox equivalents (TE) per 100 g of fresh fruit weight (FW).
Statistical analysis
Physicochemical and bioactive composition assays were performed in triplicate and antioxidant determination techniques in quadruplicate for each sample. All data are reported as mean ± standard deviation of replicates. The means of all evaluated parameters were compared using Infostat Software (Di Rienzo, et al., 2017). Analysis of variance (ANOVA) and Tukey test were carried out to identify significant differences between different species or varieties with a significance at p < 0,05. Relationships between phenolic compounds content and antioxidant capacity were determined with the Pearson correlation analysis with the same statistical program.
Results and discussion
Physicochemical characterization
According to the results of physicochemical parameters (Table 1) the fruits evaluated were suitable for fresh consumption. Selections of ‘Arazá’ species were grouped into ‘Arazá Amarillo’ (AA - P. cattleyanum f. lucidum) and ‘Arazá Rojo’ (AR - P. cattleyanum f. cattleyanum) in accordance with the botanical forms and differences in their skin color. Differences found for this fruit were also related to the results of fruit weight, pH, titratable acidity (TA) and total soluble solids (SS), being comparable with previous studies (Medina, et al., 2011; dos Santos Pereira, et al., 2018).
Table 1. Physicochemical characterization in native fruits: AA - Arazá Amarillo; AR - Arazá Rojo; GI - Guaviyú and GO - Guayabo del país. Data are expressed as mean values ± standard deviation; nd = not determined. Different letters within rows indicate significant differences at p < 0,05 by the Tukey test.
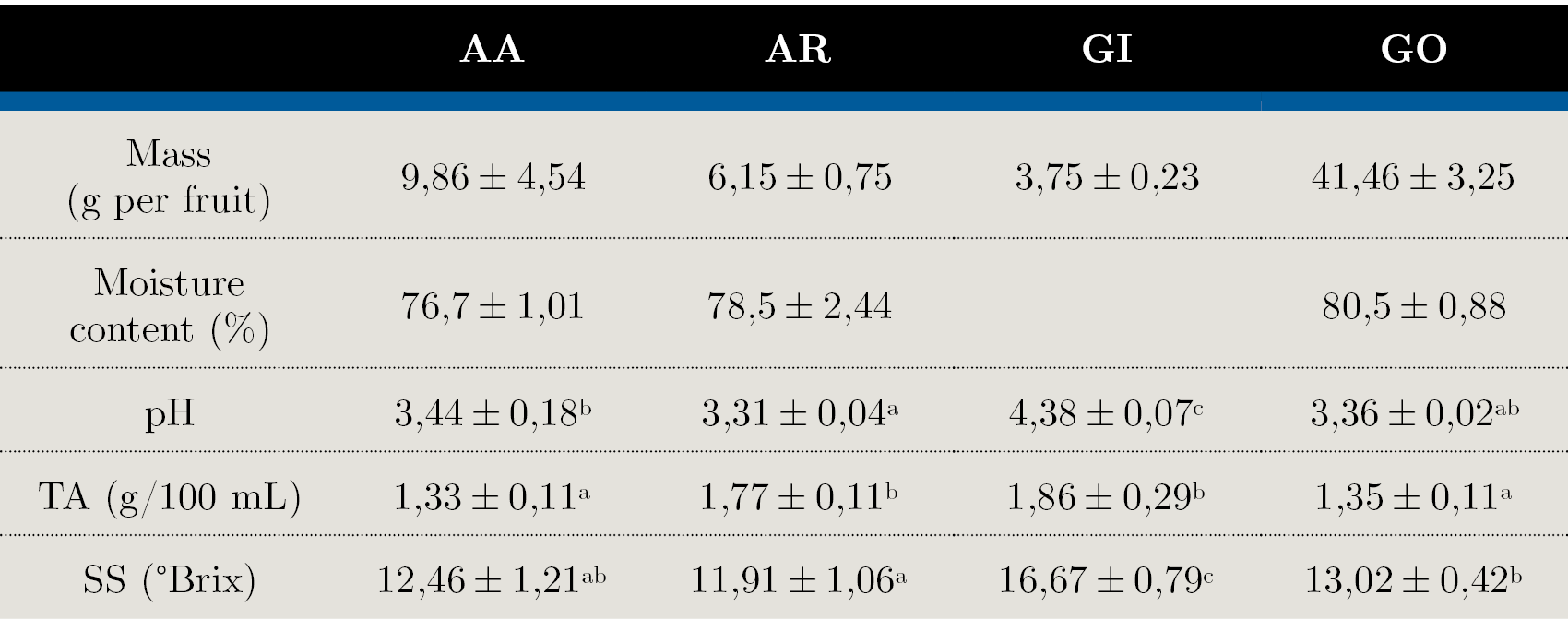
‘Guayabo del país’ (GO) were larger in size and displayed greater fresh mass than the other fruits. The average weight of all evaluated fruits varied greatly among cultivars, mainly in AA selections. These variations in the fruit weight were mainly influenced by the genotype but also by the soil, climatic conditions and fruit maturity stage. Since the climatic and soil conditions were the same for all the cultivars evaluated in our study, the variability observed may be due to genetic differences among selections.
The highest values of pH, TA and SS content were found in ‘Guaviyú’ (GI) fruit. The SS:TA ratio is one of the most important parameters to determine the taste and palatability of the fruit and is correlated with consumer acceptability (Crisosto and Crisosto, 2005). In the evaluated fruits this ratio was significantly low for AR, indicating the predominance of acid on sweet taste, confirmed by the high TA and low SS values.
Bioactive compounds
The results for anthocyanins, vitamin C and total phenolic compounds are shown in Table 2. Anthocyanins concentration varied from 0,18 to 45,90 mg of c3g/100 g FW. GI presented the highest anthocyanins content, comparable to those of other well-known fruits sources of anthocyanins (Wu, et al., 2006) as to other Myrtaceae fruits (Rufino, et al., 2010). Anthocyanins are responsible for the red, blue and purple pigmentation in the fruits and, as expected, they were present in very low concentrations in AA and GO fruit flesh. The content of anthocyanins in the AA and AR selections varied significantly between 0,18 ± 0,16 to 8,13 ± 0,04 mg of c3g/100 g FW, which is consistent with results found in other studies (Vinholes, et al., 2017).
Table 2. Bioactive compounds content in the evaluated native fruits: AA - Arazá Amarillo; AR - Arazá Rojo; GI – Guaviyú and GO - Guayabo del país. Data are expressed as mean values ± standard deviation; nd = not determined. Different letters within rows indicate significant differences at p < 0,05 by the Tukey test.
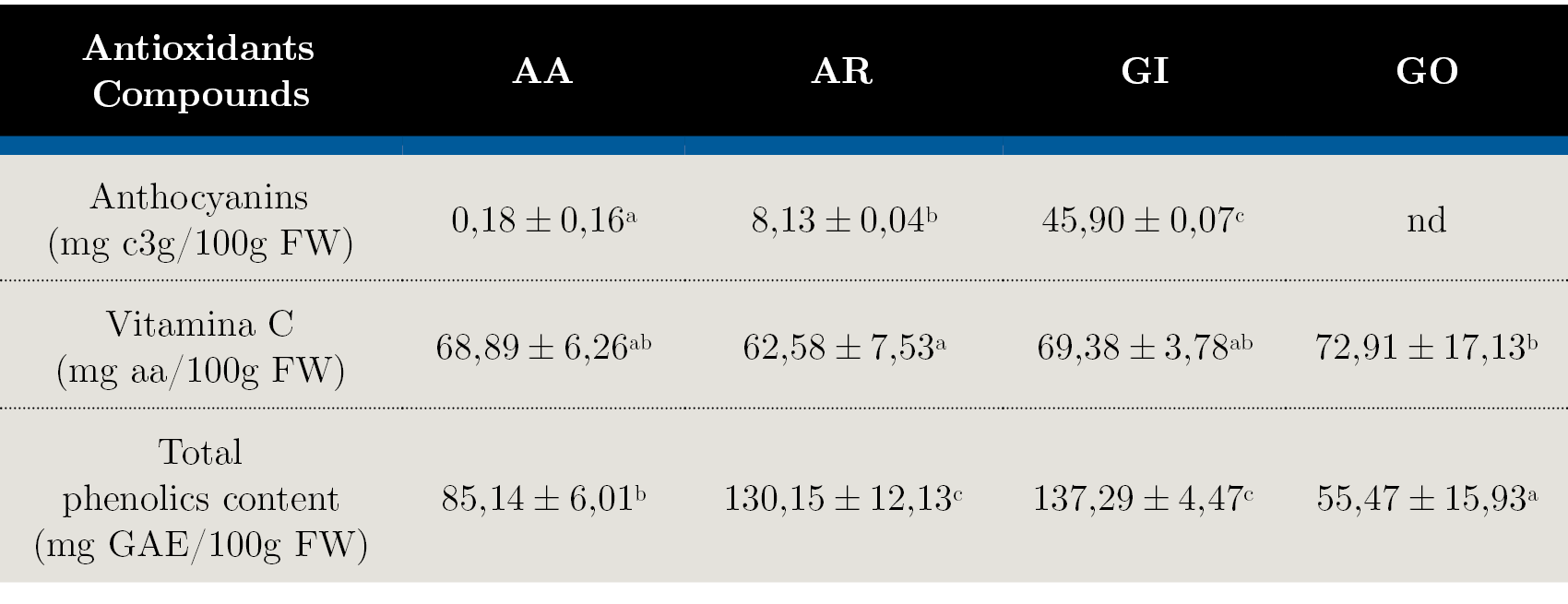
The average vitamin C content was 68,44 mg aa/100 g FW with significantly differences between the evaluated fruits. Similar results were reported for fruits and vegetables like red pepper, orange, grapefruit and kiwifruit which are considered the main contributors of vitamin C in the human diet (National Institutes of Health, 2018). This fruits therefore could be considered as good sources of this vitamin for human nutrition purpose (dos Santos Pereira, et al., 2018). It is well known that ascorbic acid levels are dependent of a wide variety of environmental factors including light, temperature, salt and drought stress, the presence of atmospheric pollutants, metals and herbicides (Davey, et al., 2000). However in our experimental conditions the differences found between selections for the same species could be explained mainly by genetic factors.
Total phenolic compounds are important for antioxidant capacity due to hydroxyl groups in their structure that give an important scavenging activity of radical species. As shown in Table 2, significant difference (p < 0,05) between total phenolic contents in the studied fruits was observed. The highest amount of phenols was determined for AR (130,15 ± 12,13 mg GAE/100g FW) and GI (137,29 ± 4,47) and the least for GO (55,47 ± 15,93). Same differences between AA and AR fruits phenolic contents were found by other authors (Medina, et al., 2011). The range of values was different from those reported in the literature (Denardin, et al., 2015; Zhu, 2018), but it may be due to diverse factors: geographic location, soil type, ambient and culture conditions and genetic variability, among others. In addition, the part of the fruit utilized, the extraction and determination methods can also be responsible for the observed differences. Most of the studies used the whole fruit, but some of them (Medina, et al., 2011) evaluated the composition on the fruit flesh. For the method of extraction, some authors use a multi-step extraction procedure including long periods of maceration with solvents with different polarities and removal of fat soluble substances prior phenolic contents analysis, while others use more simple methods (dos Santos Pereira, et al., 2018). When compared with local results found for these native fruits, we obtained relative higher ascorbic acid content and lower total polyphenol content (Feippe, et al., 2011; Silveira, et al., 2016).
Antioxidant activity
Table 3 shows the antioxidant activity determined by DPPH and ORAC assays. The antioxidant capacity displayed in native fruits varied according to the species analyzed (p < 0,05). The AA, AR and GI fruits have the highest DPPH values, more than two times the activity observed in GO (1560,2 ± 792,4 mmol TE/100g FW).
Table 3. Antioxidant activity determined by DPPH and ORAC assays in native fruits: AA - Arazá Amarillo; AR - Arazá Rojo; GI - Guaviyú and GO - Guayabo del país. Data are expressed as mean values ± standard deviation. Different letters within rows indicate significant differences at p < 0,05 by the Tukey test.
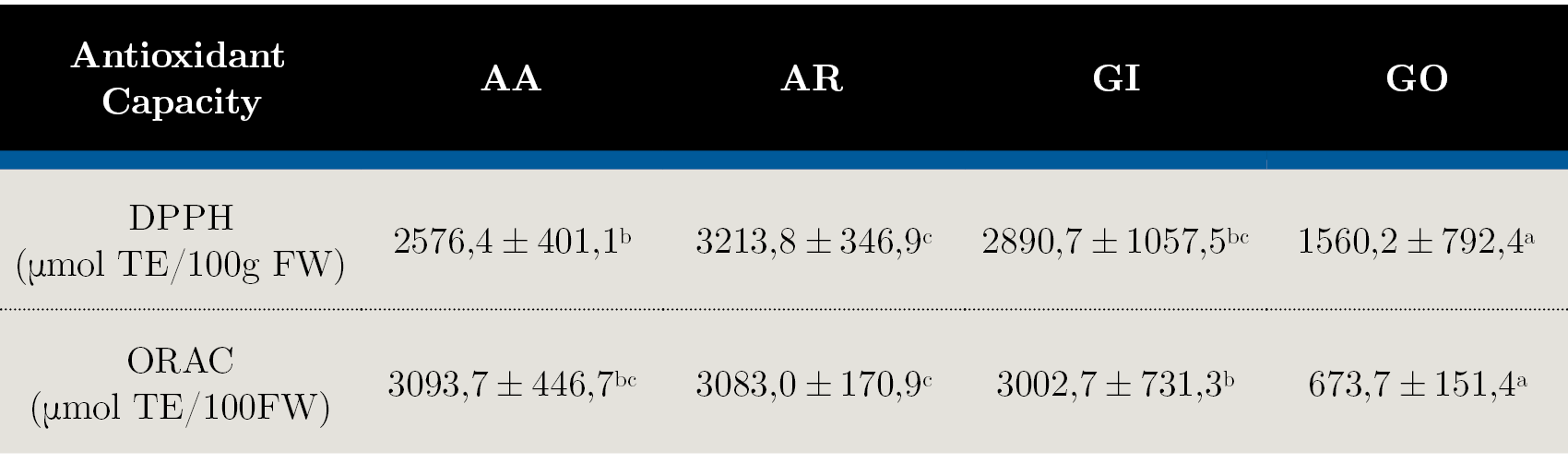
Among the ‘Arazá’ fruit, AR selections exhibited higher DPPH activity than AA. Similarly, the highest ORAC values were observed for AA, AR and GI with an average of 3059,8 mmol TE/100g FW, whereas a low antioxidant activity (637,7 mmol TE/100g FW) was measured in GO fruit. The variability among selections of same fruit species were high, 28% and 16% in average to DPPH and ORAC analysis, respectively. Limited information has been reported about antioxidant capacities in the fruit considered in this study. According to Ribeiro da Silva et al. (2014) ‘Arazá’ fruit flesh is a potent scavenger of reactive oxygen and nitrogen species. Similar results were obtained by Medina et al. (2011) and Vinholes et al. (2017) where edible portions of ‘Arazá’ from yellow and red genotypes showed higher antioxidant capacity measured with DPPH. In vitro studies of AR and GI demonstrated that these fruit can be considered as a good source of bioactive compounds with antioxidant properties (Dalla Nora, et al., 2014). However, cultivar, harvest time, maturity, storage and agroclimatic factors, as well as the part of fruit tested, can be responsible for frequent variation in antioxidant capacity values observed among fruits.
High and significant correlation between DPPH and ORAC results was obtained (r = 0,80, p < 0,01), indicating that any of these assays can be suitable for the antioxidant capacity evaluation of the fruits in our analytical conditions. Additionally, significant correlations were observed between total phenolics content and DPPH (r = 0,73, p < 0,01) and ORAC (r = 0,82, p < 0,01) showing that these compounds seem to contribute to the antioxidant capacity of the studied fruits.
Conclusions
‘Arazá’ (Psidium cattleianum), ‘Guaviyú’ (Myrcianthes pungens) and ‘Guayabo del país’ (Acca sellowiana) fruits proved to be good sources of natural antioxidants such as vitamin C and phenolics compounds, with high antioxidant capacity potential. The physicochemical and antioxidant characterizations of these native fruit selections could be a valuable tool to promote their consumption and represent good perspectives for nutraceutical purposes.
The information regarding bioactive compounds and antioxidant activities is important for nutritional characterization of the native fruits, but also to evaluate genetic and agronomic performance in order to develop new fruit crops. More studies could be conducted in other research areas as commercial production, fruit postharvest, storage conditions or food processing.
